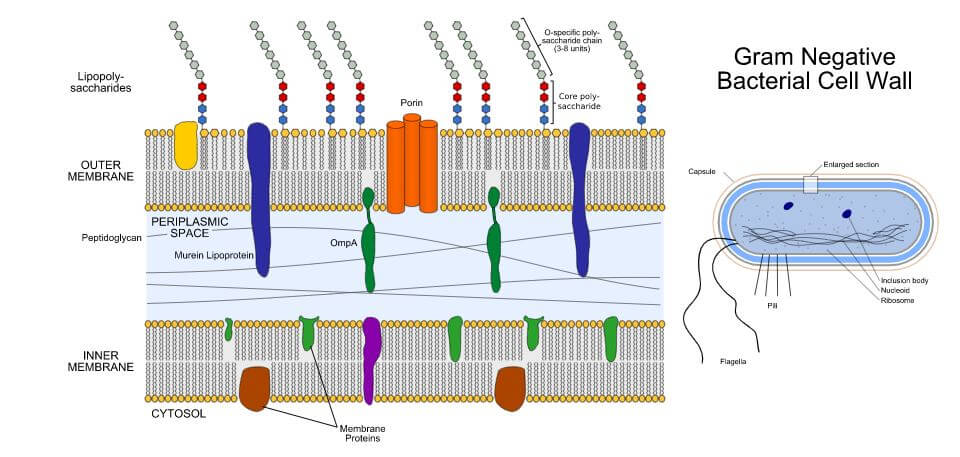
Endotoxin is the common name for the lipopolysaccharides (pictured below)* embedded in the outer membrane of all gram-negative bacteria. A gram-negative bacteria must die and break down for endotoxin to be available for reaction, making it impossible to detect with sterility testing.
Endotoxin is the common name for the lipopolysaccharides (pictured below)* embedded in the outer membrane of all gram-negative bacteria. A gram-negative bacteria must die and break down for endotoxin to be available for reaction, making it impossible to detect with sterility testing.
How Is Endotoxin Measured?
How Is Endotoxin Measured?
Endotoxin is measured in Endotoxin Units (EU). An EU is defined as the activity of 0.2 nanograms of Reference Endotoxin Standard. Due to the nature of most tests requiring a liquid medium, endotoxin specifications and results are often written in EU/mL.
The presence of endotoxin in the bloodstream is called endotoxemia. If endotoxin-contaminated blood circulates through the liver, many immune cells can release too rapidly, leading to overexpression. Overexpression of an immune response within the body can, in serious cases, lead to shock, organ failure, and death.
Ingestible medications cannot cause endotoxemia because of the acid within the digestive tract. Endotoxin can be dissolved and avoid absorption through the intestinal walls. Contaminated injectable and ophthalmic medications can cause endotoxemia because of their interaction with the bloodstream
Assessing Endotoxin
Assessing Endotoxin
Sterile processes of pharmaceutical formulation cannot prevent endotoxin contamination entirely. Sterile processes often focus on killing bacteria, if present, which leads to the expression of endotoxin. Endotoxin can be introduced at any point and by anything in the process of formulating a pharmaceutical product, from the solvent water to the raw materials of the packaging.
The three Bacterial Endotoxin Tests (BET) that use Limulus Amoebocyte Lysate (LAL) are gel-clot assay, chromogenic, and kinetic turbidimetric testing. Infinity Laboratories offers all these methods of testing.
Limulus amoebocytes form coagulin when in the presence of endotoxin. During the gel-clot assay test, LAL reagent is introduced to a sample tube, and endotoxin is present if the sample coagulates into a clot or gel-like mass. Gel-clot assay is a qualitative test; it does not specify the amount of endotoxin present, only that it is or is not. This quick pass/fail test is available at the Crown Point testing facility.
Kinetic turbidimetric testing is the quantitative collection of coagulation data. The process is sensitive enough to detect down to 0.005EU/mL. The samples are tested in duplicate to collect quantitative coagulation data and compared to a standard curve. Each sample is tested unchanged and with a known amount of endotoxin in a 96-well microplate. The microplate is dosed with lysate, and the wells with endotoxin will coagulate. The microplate reader will measure the degree of coagulation. Kinetic turbidimetric testing is offered at the Infinity sites in San Diego, St Louis, and Crown Point.
The reaction that creates coagulin also breaks bonds between proteins and a specific dye. Once the dye is free of the protein, it can express its yellow color. This color is used in chromogenic testing to determine if endotoxin is present. Chromogenic testing can be qualitative and quantitative, depending on the number of data points taken.
Chromogenic endpoint testing is qualitative, with only one data point being recorded. Kinetic chromogenic testing takes multiple data points during the test, allowing for a quantitative result. Kinetic chromogenic testing is sensitive enough to detect down to 0.005EU/mL when done via cartridge or microplate testing. Kinetic chromogenic testing is offered at the Crown Point, Petaluma, and San Diego Infinity Laboratory locations.
References
References
1. Su W, Ding X. Methods of Endotoxin Detection. Journal of Laboratory Automation. 2015;20(4):354-364. doi:10.1177/2211068215572136. https://journals.sagepub.com/doi/10.1177/2211068215572136?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
2. Dept. of Health, Education, and Welfare Public Health Service Food and Drug Administration *ORA/ORO/DEIO/IB*. Bacterial Endotoxins/Pyrogens. Inspection Technical Guides. 2014.
3. Charles River. Gel-Clot Endotoxin Test. https://www.criver.com/products-services/qc-microbial-solutions/endotoxin-testing/lal-reagents-accessories/gel-clot-lal?region=3611
4. Fujifilm Pyrostar. Use of Colorimetric Method in LAL Test. 2018. https://www.wakopyrostar.com/blog/kit-lal/post/use-of-colorimetric-method-in-lal-test/ – :~:text=The colorimetric analysis methods allow,Limulus amebocyte lysate) for endotoxins