With the rapid development of biologics produced via cell substrates, keeping on top of your aseptic procedures and routine testing is paramount to ensure your production batch isn’t botched.
With the rapid development of biologics produced via cell substrates, keeping on top of your aseptic procedures and routine testing is paramount to ensure your production batch isn’t botched.
What is mycoplasma contamination and why does it matter?
What is mycoplasma contamination and why does it matter?
Mycoplasma is the smallest prokaryotic organism that can live in cell-free culture media. Because they lack a cell wall, mycoplasma is resistant to the most common antibiotics, and they may pass through the conventional 0.2 μm filters used in cell culture, thus raising the potential for high contamination rates. Contamination can alter virtually every chemical and physical property of cells (depending on the contaminating species and the host type), causing them to yield unreliable results and perhaps unsafe biologicals, biopharmaceutical drugs, or viral vaccines. In addition, mycoplasma contamination can be present with no obvious change in the host culture, making it very challenging to detect without dedicated testing. Whether your product is derived from cell substrates or is used by your customers in cell culture, nobody wants mycoplasma in their facility.
What are the sources of mycoplasma contamination?
What are the sources of mycoplasma contamination?
Over 200 mycoplasma species are identified, originating from many sources ranging from soil to mammals, cells, and insects.
Laboratory Personnel: Did you know that most mycoplasma contamination comes from people? Many mycoplasma species are found on human skin and saliva (M. hominis, M. orale, and M. fermentans to name a few). A simple cough, sneeze, or even talking without a mask can inadvertently introduce mycoplasma to surfaces you do not want it to be. Due to their extremely small size, mycoplasma cannot be detected in routine Viable Environmental Monitoring of your cleanroom space or Biosafety Cabinet.
Raw Materials: Contaminated animal-derived supplements like fetal bovine serum and trypsin can lead to a full-scale contamination. Other reagents, such as plant-based serums, media, and feeder cells, are also susceptible to contamination. Ensure you have a robust material receiving system that includes receipt of C of As, qualified vendors, and testing available to ensure all your raw materials are mycoplasma-free.
Equipment: Bioreactors, tubing, water baths, liquid nitrogen, even pipettes can harbor mycoplasma. These can all be considered potential culprits when testing and troubleshooting cultures. Ensure that all equipment is cleaned adequately and frequently. Adopt a robust and validated Cleaning and Disinfection Program for your cleanrooms and work surfaces.
You may never know if you have a mycoplasma contamination unless you test for it. Demonstrating that your product is free of adventitious agents (like mycoplasma) is not only a good checkpoint in your process, it’s also a regulatory requirement.
What can I do to prevent mycoplasma contamination at my site?
What can I do to prevent mycoplasma contamination at my site?
Strict adherence to Aseptic Technique procedures: It is essential to have well-trained, accountable personnel working in aseptic conditions. Limit talking in controlled environments, never pipette by mouth, contain all work in a Biosafety Cabinet, and maintain strict adherence to gowning procedures. Working in closed systems is also recommended, but remember, there will always be some form of human exposure to the raw materials and intermediates, so stay vigilant and work carefully.
Trust your sources: Can you be sure that the companies that provide your primary components are doing their due diligence? Many media and supplement producers will test each batch of their products for mycoplasma. If this data is unavailable or your Quality department requires third-party testing, include routine testing for mycoplasma as a requirement for releasing or green-tagging raw materials such as media, serum, or enzymes for your process.
Test Frequently: You may never know if you have a mycoplasma contamination unless you test for it. Demonstrating that your product is free of adventitious agents (like mycoplasma) is not only a good checkpoint in your process, it’s also a regulatory requirement. Let’s take a look at the test points in a generic cell culture process where samples can be tested for mycoplasma. The further upstream you perform your testing, the earlier you know and can mitigate any contamination to preserve labor and material costs.
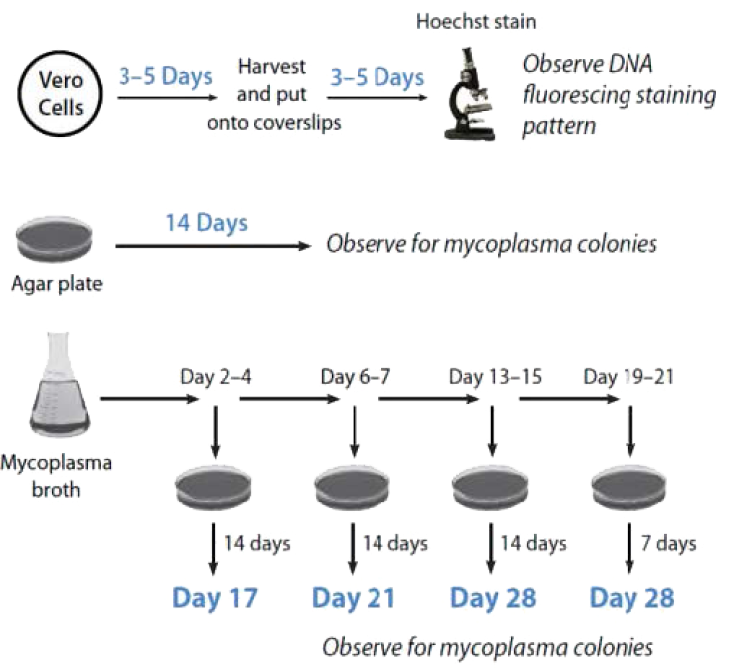
Several traditional test methods follow USP, EU, and FDA Standards. This series of testing, known as direct and indirect culture methods, can be cumbersome due to the long incubation times and reliance on human observance for the growth of the smallest free-living organism known to man. In the age of accelerated timelines to push product out the door, are you prepared to quarantine your raw materials or product for a month before resuming production? Time is money, right?
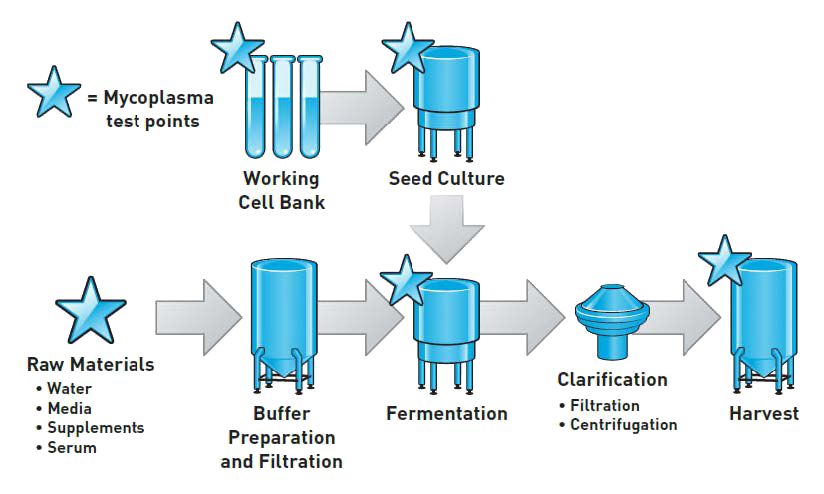
In 2007, the European Pharmacopeia accepted nucleic acid tests, such as real-time PCR, as an alternative method for traditional mycoplasma detection after validation. PCR-based detection methods are highly sensitive and can provide rapid results, allowing researchers to respond quickly to isolate and eliminate contamination once it is detected compared to the time required using traditional techniques.
At Infinity Laboratories, Inc., we employ the MycoSEQ® mycoplasma detection system from Thermo Fisher Scientific, which was designed to meet the guidance provided by the European Pharmacopoeia in 2007 of rapid-response tests. MycoSEQ, which utilizes quantitative PCR (qPCR) to detect more than 90 Mycoplasma species, can provide results in hours rather than 10 to 28 days. Using state-of-the-art automated equipment, mycoplasma DNA is extracted by magnetic beads (also known as Solid Phase DNA Extraction) and then amplified in a thermal cycling machine. With the aid of SYBR® Green fluorescence technology, amplified DNA can be detected in real-time to determine whether mycoplasma is present or absent in a sample.